PharmaKure granted MHRA Clinical Trial Authorisation (CTA) for PK051 for the treatment of mild cognitive impairment due to Alzheimer’s Disease
PharmaKure, a clinical stage pharmaceutical company spun out from the University of Manchester developing precision medicines for Alzheimer’s Disease and other neurodegenerative diseases, today announces that the UK Medicines and Healthcare Regulatory Agency (MHRA) has granted Clinical Trial Authorisation (CTA) for PharmaKure to commence a muti-ascending dose Phase 2a study to evaluate safety and tolerability of PK051 intended for the treatment of mild cognitive impairment (MCI).
PK051 is an oral combined drug that targets disaggregation of amyloid-β proteins. There is increasing scientific acceptance that overproduction and/or deposition of amyloid-β is the initial event in Alzheimer’s Disease pathology.
“The MHRA authorisation marks a major step forward in our mission to develop PK051 as a disease modifying therapy for MCI due to Alzheimer’s Disease,” – Dr Farid Khan, CEO of PharmaKure.
He adds “This authorisation follows successful study results recently announced by the Company for a novel whole blood test to quantify Alzheimer’s Disease biomarkers. PharmaKure’s proprietary ALZmetrixTM blood test can identify blood-based biomarkers in patients with Alzheimer’s Disease to provide early warning of cognitive decline. Used as a companion diagnostic, this could enable treatments such as PK051 to be offered earlier to provide better population-based health outcomes.”
Dr Bob Smith, Chief Clinical Director, PharmaKure Limited, commented, “We are delighted to have approval to begin clinical testing of PK051. This Phase 2a study is intended to confirm safety, tolerability and to help us determine an appropriate dose for future efficacy studies. The trial will involve 40 patients with MCI due to Alzheimer’s Disease at a single site in the UK. The first patient is expected to be dosed in early to mid 2024, with preliminary clinical data emerging within 12 months of first dose.”
About Alzheimer’s Disease
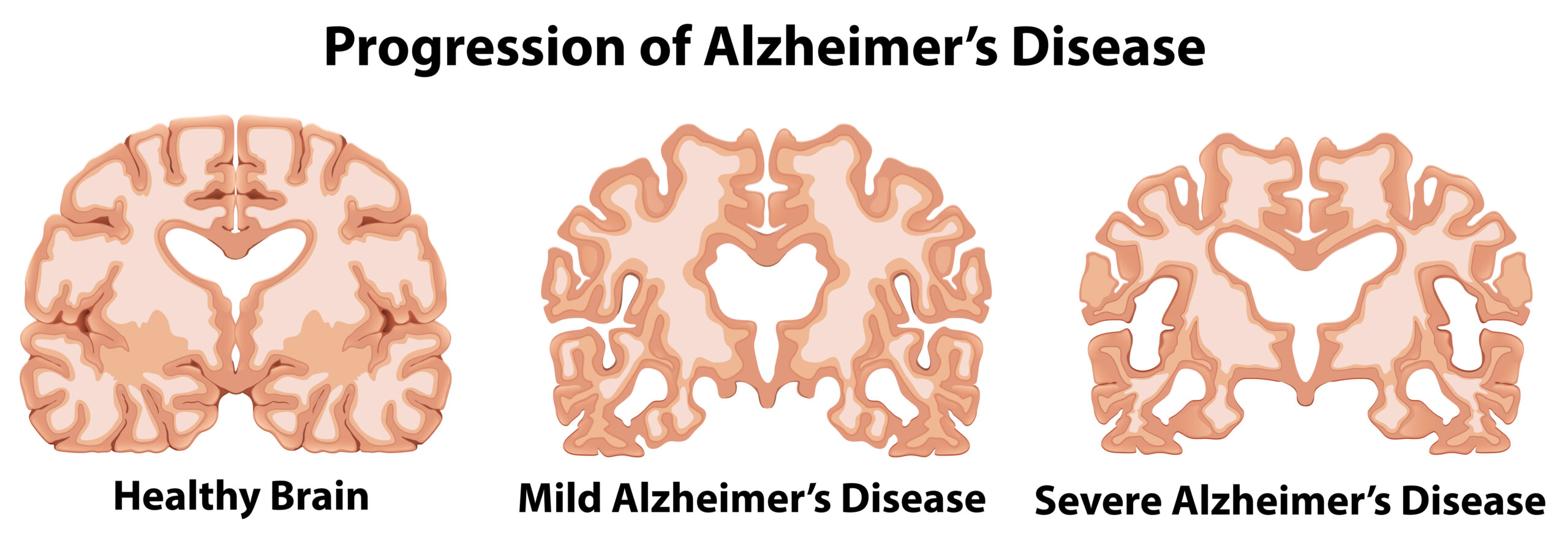
Alzheimer’s disease is a fatal illness causing a progressive decline in memory and cognition. It accounts for 60 to 80 percent of all dementia cases, affecting 850,000 people in the UK and 44 million people worldwide. The global cost of care was $360 billion in 2020, projected to reach $1 trillion by 2050 unless effective interventions are found.
About PharmaKure: PharmaKure, a clinical-stage pharmaceutical company originating from the University of Manchester, is actively researching, developing, and repositioning repurposed drugs for Alzheimer’s, Parkinson’s, and other neurodegenerative diseases. In addition to drug development, PharmaKure is advancing its companion diagnostic, ALZmetrixTM.

For more information, contact info@pharmakure.com or visit PharmaKure’s website.