Revolutionising Neurodegenerative Disease Treatment: Precision Medicines for Alzheimer’s and Parkinson’s

In the relentless pursuit of finding effective treatments for neurodegenerative diseases, PharmaKure, a clinical-stage pharmaceutical spinout company from the University of Manchester, is making remarkable strides. Specialising in the development, commercialisation and repositioning of repurposed drugs, PharmaKure is at the forefront of combatting debilitating conditions such as Alzheimer’s, Parkinson’s, and other neurodegenerative disorders.
A Growing Crisis: Alzheimer’s and Parkinson’s Disease
Alzheimer’s and Parkinson’s diseases have reached alarming levels, affecting millions of individuals worldwide. These conditions not only rob individuals of their cognitive and motor functions but also take an enormous toll on families and healthcare systems. To put it into perspective:
- Alzheimer’s disease is the most common cause of dementia, accounting for approximately 60-70% of cases. Globally, around 55 million people are living with dementia, and this number is expected to triple by 2050 if effective treatments are not found.
- Parkinson’s disease affects over 10 million people worldwide. It’s a progressive neurological disorder that primarily impairs motor function, but it can also cause cognitive decline and a range of other symptoms.
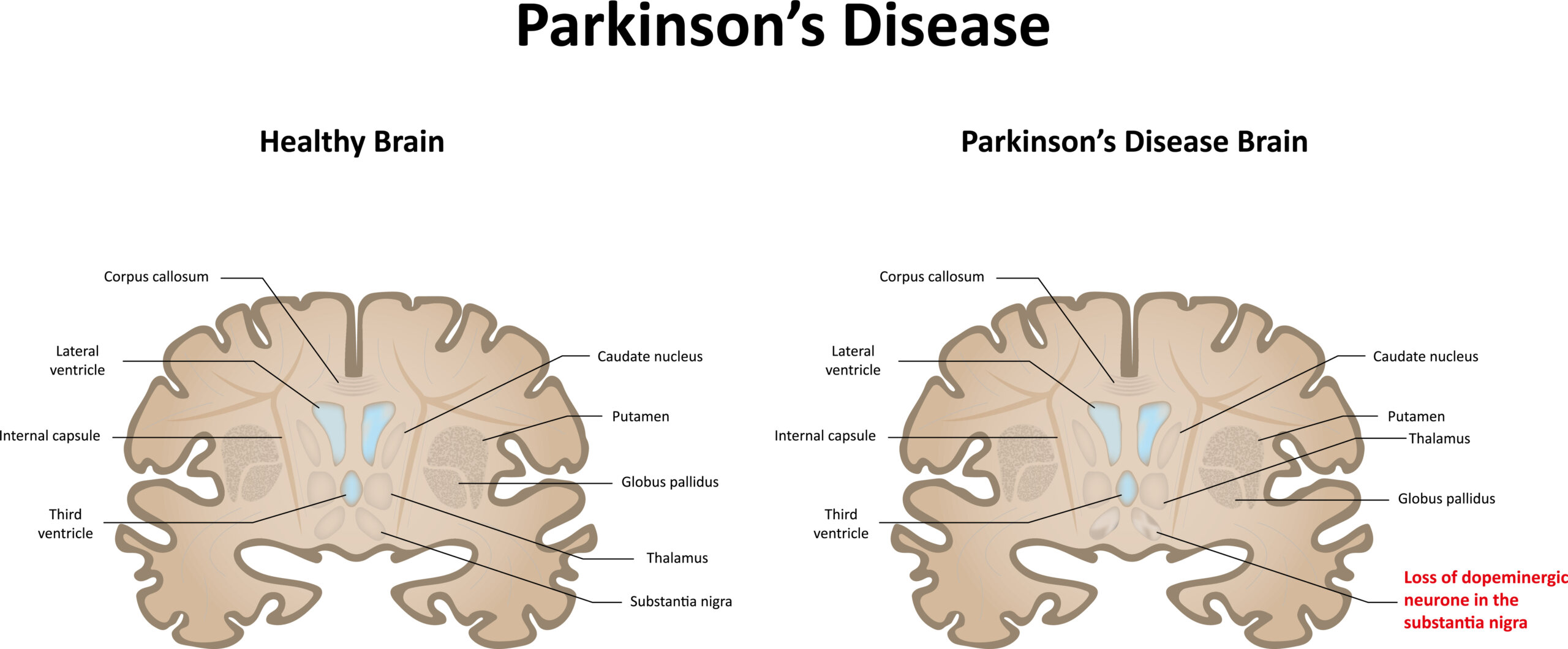
Illustration comparing healthy brain to a brain with Parkinson’s
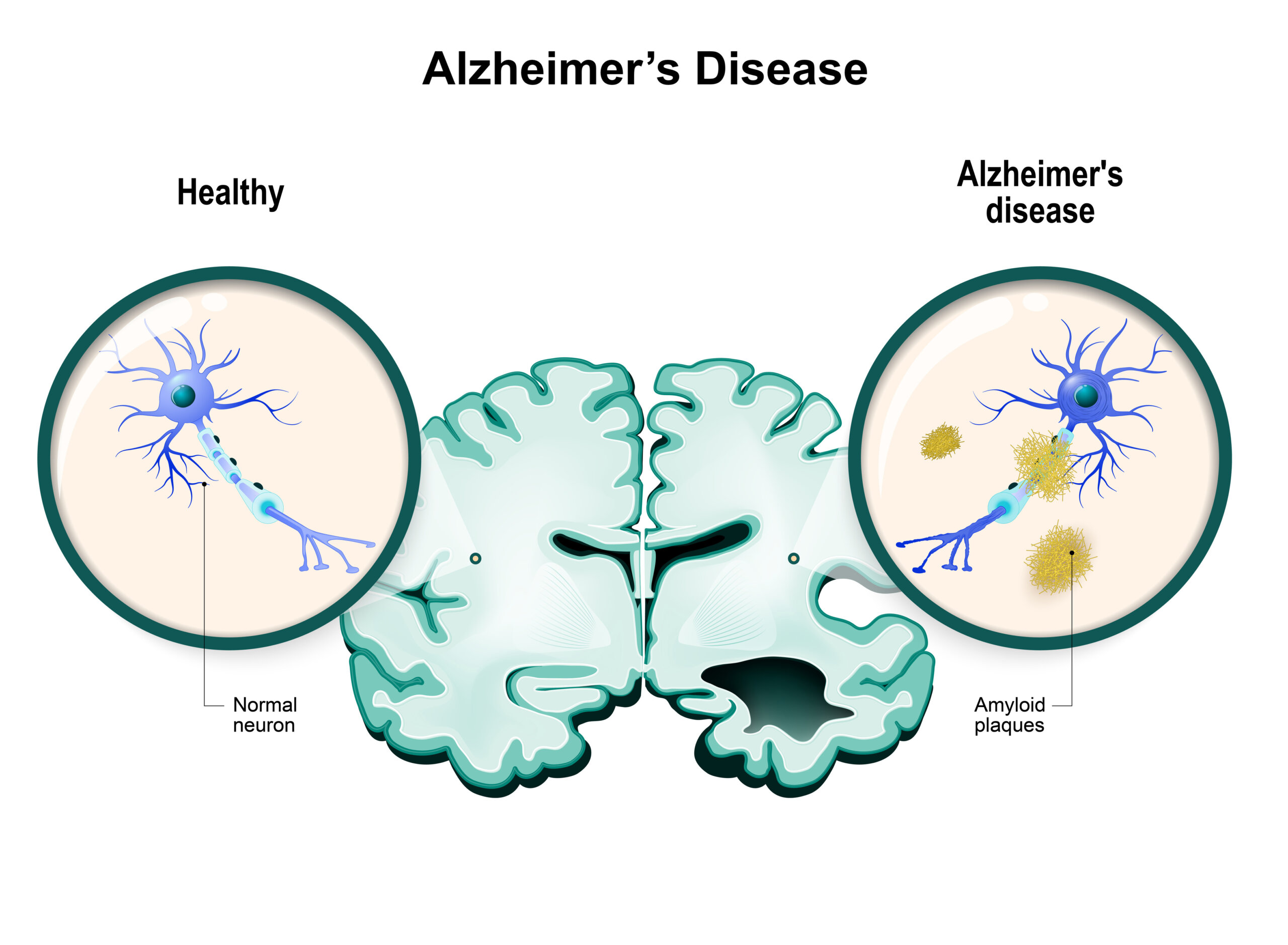
Illustration comparing a healthy human brain and a brain with Alzheimer’s disease. Shows a healthy neuron and neuron with amyloid plaques.
PharmaKure’s Innovative Approach
How does PharmaKure’s groundbreaking approach tackle these devastating diseases? PharmaKure’s drugs are repurposed small molecules, administered orally, and they act as ‘dis-aggregators’ of neurotoxic proteins. This innovative strategy holds promise in slowing down or even halting the progression of these diseases, offering hope to patients and their families.
But PharmaKure doesn’t stop at drug development. They recognise the urgent need for cost-effective, non-invasive diagnostic tests for neurodegenerative diseases. Current methods, such as MRI or PET scans, can be prohibitively expensive and invasive.
To address this challenge, PharmaKure has developed companion diagnostic biomarker assays called ALZmetrixTM and PARKmetrixTM. These advanced blood tests can measure the levels of neurotoxic oligomeric proteins in red blood cells for Alzheimer’s and Parkinson’s diseases, respectively. This breakthrough not only simplifies the diagnostic process but also makes it more accessible to a broader range of patients.
Expanding the Horizon
PharmaKure’s ambition extends beyond their innovative drugs and diagnostic tools. Their business model includes potential revenue generation from blood-based biomarkers, which can be used to stratify patients for clinical trials in various neurodegenerative diseases, including Alzheimer’s Disease, Parkinson’s Disease, Dementia with Lewy Bodies, Parkinson’s with dementia, Amyotrophic Lateral Sclerosis, and Multiple Systems Atrophy.
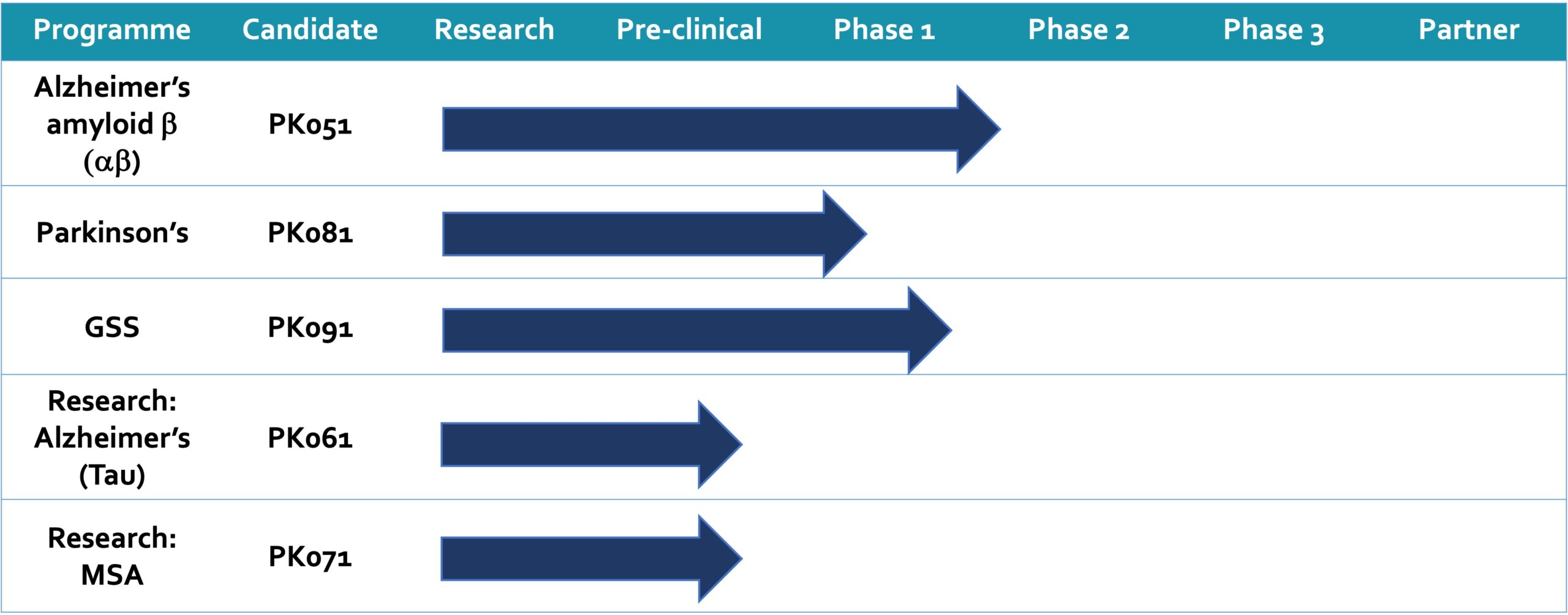
Their impressive portfolio of granted patents covers a spectrum of neurodegenerative diseases related to PK051, PK081, and PK091 in the US and Europe. Currently, clinical studies are underway for the ALZmetrixTM biomarker assay, focusing on early detection of Alzheimer’s disease.
Additionally, PharmaKure is gearing up for a clinical Phase 2a study for their drug candidate PK051, targeting Alzheimer’s Disease, set to commence in 2023. Simultaneously, the PARKmetrixTM biomarker assay, designed for early detection of Parkinson’s disease, is also slated to begin its study in the same year.
A Team of Experts
Behind these remarkable achievements stands an experienced team of clinical trial experts, clinicians, drug and assay scientists, and leading academics from The University of Manchester and The University of Cambridge. Their collective expertise ensures that PharmaKure’s research and development efforts are grounded in the latest scientific knowledge and industry best practices. You can meet the PharmaKure team here
Opportunities
PharmaKure is on a mission to transform the landscape of neurodegenerative disease treatment and diagnosis. They are actively seeking investors, licensing partners, and co-development collaborators, including national health centers, to accelerate their clinical diagnostic and drug programs. Please get in touch with the project manager for more information.
Sources for statistics: