PharmaKure submits MHRA CTA for Phase 2a Clinical Trial of PK051 in patients with mild cognitive impairment

In a significant stride toward combatting Alzheimer’s Disease, PharmaKure, a clinical stage pharmaceutical company, and one of our esteemed spinouts, has submitted a Clinical Trial Application (CTA) to the Medicines and Healthcare Regulatory Agency (MHRA) in the United Kingdom. This application marks the next chapter in the journey of PK051, a combined drug that targets disaggregates of amyloid-β proteins, a key factor in Alzheimer’s Disease pathology.
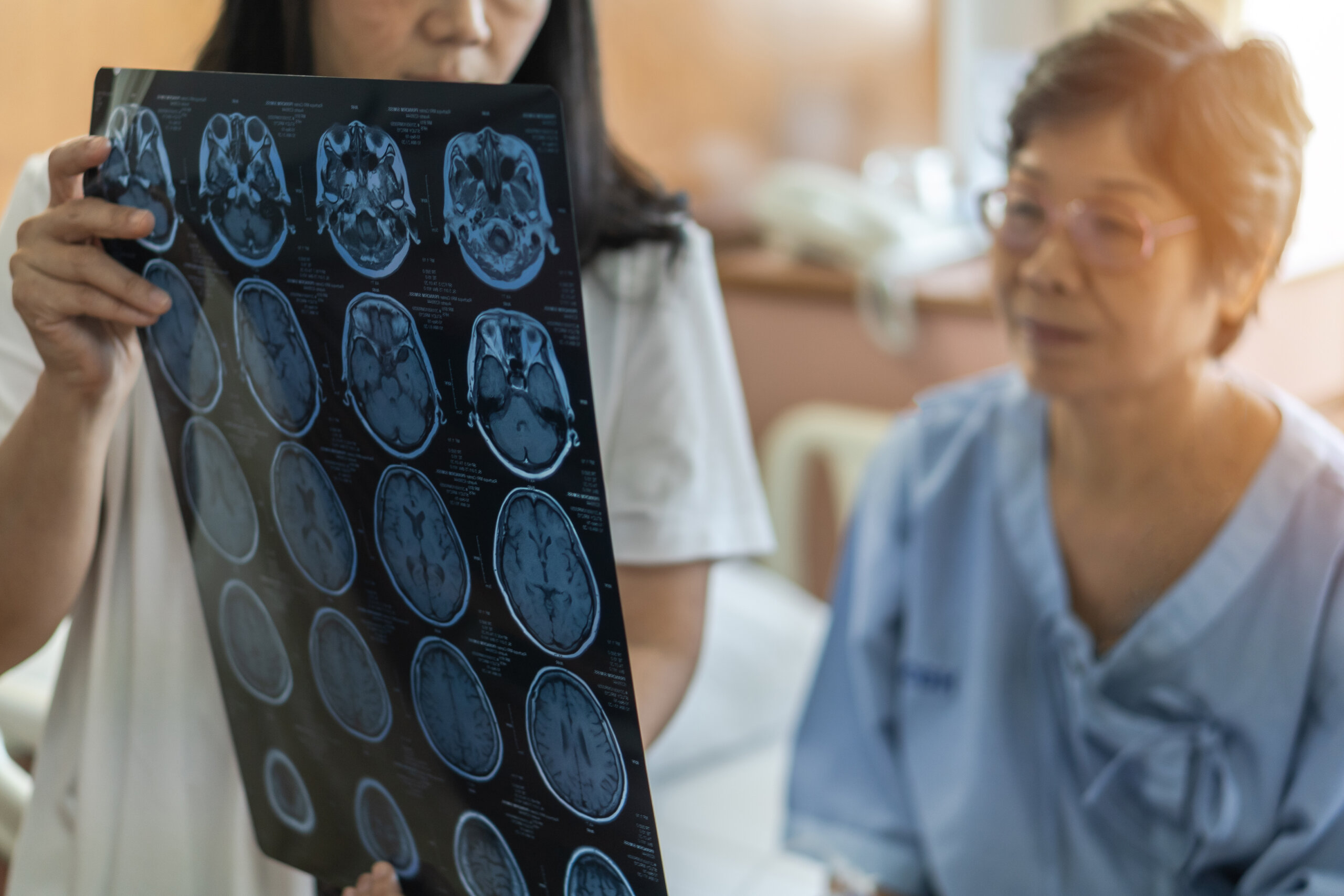
MRI scans can indicate whether people are developing different types of dementia, and reveal how people are responding to treatments
PharmaKure, is at the forefront of precision medicine development for Alzheimer’s Disease and other neurodegenerative conditions. The CTA submission includes comprehensive data from pre-clinical studies, rigorous safety assessments, and a robust clinical trial protocol, emphasising PharmaKure’s commitment to the highest standards of scientific and ethical conduct throughout the clinical trial process.
Dr. Farid Khan, CEO of PharmaKure, expressed the company’s dedication to advancing Alzheimer’s Disease research, saying, “We believe that PK051 has the potential to significantly improve the quality of life for patients. Our team’s tireless efforts have brought us to this milestone, and we are eager to collaborate with the MHRA and other stakeholders to bring PK051 one step closer to those in need.”

Dr. Farid Khan, Director and Co-Founder of PharmaKure
Upon MHRA approval, PharmaKure plans to initiate a phase 2a clinical trial, assessing the multi-ascending dose, safety, and tolerability of PK051. This trial will involve 40 patients at a single site in Motherwell, UK, with the first patient expected to be dosed in early 2024, and clinical data emerging within 12 months of the first dose.
This achievement follows PharmaKure’s recent success in developing a novel whole blood test, ALZmetrixTM, which quantifies Alzheimer’s Disease biomarkers. ALZmetrixTM has the potential to offer early warning of cognitive decline and improve population-based health outcomes when used as a companion diagnostic with treatments like PK051.
About Alzheimer’s Disease – Alzheimer’s Disease is a devastating illness that leads to progressive decline in memory and cognitive function. It is the most common form of dementia, accounting for a substantial portion of dementia cases worldwide.
About PharmaKure – PharmaKure is a clinical stage pharmaceutical company that emerged from the University of Manchester. They are actively engaged in research, development, and commercialisation of drugs for the treatment of Alzheimer’s, Parkinson’s, and other neurodegenerative diseases, alongside the development of their companion diagnostic, ALZmetrixTM.
For more information, please contact:
- Dr. Farid Khan, CEO: farid.khan@pharmakure.com
- Dr. Bob Smith, Clinical Director: robert.smith@pharmakure.com
- Dr. Naz Bashir, CCO: naz.bashir@pharmakure.com



